

One corneal implant segment is introduced to each channel. An incision is made in the cornea and channels are created in it by rotating a lamellar dissector or by using a femtosecond laser. The procedure is undertaken under local or general anaesthesia.
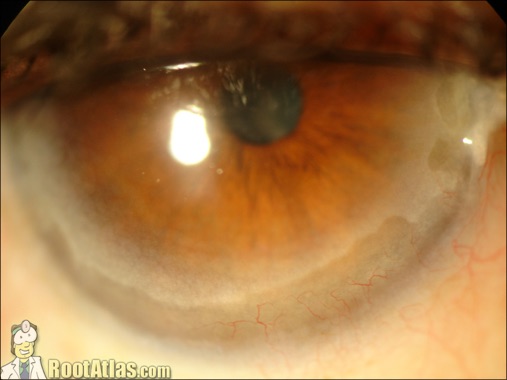
They affect refraction in the eye by physically changing the shape of the cornea, flattening the front of the eye. Ultimately a corneal transplant may be required in some patients.Ĭorneal implants are flexible, crescent-shaped rings of polymethyl methacrylate that are placed in the periphery of the cornea. Invasive procedures include penetrating keratoplasty to modify the shape of the cornea. In more severe disease, other treatments may include collagen cross-linking riboflavin eye drops. In mild keratoconus, spectacles or soft contact lenses may help. The cornea within and adjacent to the thinned area is ectatic. It is not known whether pellucid marginal degeneration and keratoconus are distinct diseases or different manifestations of the same disorder. This procedure can also be used for pellucid marginal degeneration: a non-inflammatory, peripheral corneal thinning disorder characterised by thinning of the peripheral band of the inferior cornea. Patients should have clear central corneas in order for them to be suitable candidates for the insertion of corneal implants. Keratoconus is often associated with astigmatism. This changes the normal physical properties of the cornea and affects refraction. Keratoconus is a progressive disease in which the normally round corneal surface becomes thinner and begins to bulge into a cone like shape. The recommendations are provisional and may change after consultation.Ĭurrent evidence on the safety and efficacy of corneal implants for keratoconus appears adequate to support the use of this procedure provided that normal arrangements are in place for consent, audit and clinical governance. Note that this document is not the Institute's guidance on this procedure. Target date for publication of guidance: July 2007 The Advisory Committee will then prepare draft guidance which will be the basis for the Institute's guidance on the use of the procedure in the NHS in England, Wales, Scotland and Northern Ireland.įor further details, see the Interventional Procedures Programme manual, which is available from the Institute's website ( Closing date for comments: 24 April 2007.The Advisory Committee will meet again to consider the original evidence and its provisional recommendations in the light of the comments received during consultation.The process that the Institute will follow after the consultation period ends is as follows. The recommendations are provisional and may change after consultation. Note that this document is not the Institute's formal guidance on this procedure. the identification of factual inaccuracies.comments on the preliminary recommendations.The Advisory Committee particularly welcomes: It has been prepared for public consultation. This document summarises the procedure and sets out the provisional recommendations made by the Advisory Committee. The Advisory Committee has made provisional recommendations about The Institute's Interventional Procedures Advisory Committee has considered the available evidence and the views of Specialist Advisers, who are consultants with knowledge of the procedure. The National Institute for Health and Clinical Excellence is examining corneal implants for keratoconus and will publish guidance on its safety and efficacy to the NHS in England, Wales, Scotland and Northern Ireland. The insertion of clear plastic implants into the cornea is an interventional procedure aiming to restore eyesight in patients with this condition. Keratoconus is a disease of the cornea which affects the shape of the eyeball and causes refractive errors, some of which cannot be corrected by spectacles or contact lenses.
Interventional Procedure Consultation Document NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE


 0 kommentar(er)
0 kommentar(er)
